-
新版TCGAbiolinks包的可视化功能
使用
TCGAbiolinks包探索最新版TCGA数据库已经写了近10篇推文。今天学习下
TCGAbiolinks包中的可视化函数。这些图形都可以使用其他R包进行更好看的可视化,平常大家根本不用,不过作为
TCGAbiolinks包完整学习的一部分,在这里简单记录一下。。library(TCGAbiolinks) suppressMessages(library(SummarizedExperiment))- 1
- 2
热图
可视化差异基因或者差异甲基化。
使用前面推文中得到的COAD的差异基因演示。
# 获取表达矩阵 load("TCGA-mRNA/TCGA-COAD_mRNA.Rdata") se_mrna <- data[rowData(data)$gene_type == "protein_coding",] coadMatrix <- assay(se_mrna, "unstranded") coad_coroutliers <- TCGAanalyze_Preprocessing(se_mrna,cor.cut = 0.7) ## Number of outliers: 0 coadNorm <- TCGAanalyze_Normalization( tabDF = coad_coroutliers, geneInfo = geneInfoHT) ## I Need about 127 seconds for this Complete Normalization Upper Quantile [Processing 80k elements /s] ## Step 1 of 4: newSeqExpressionSet ... ## Step 2 of 4: withinLaneNormalization ... ## Step 3 of 4: betweenLaneNormalization ... ## Step 4 of 4: exprs ... coadFilt <- TCGAanalyze_Filtering( tabDF = coadNorm, method = "quantile", qnt.cut = 0.25 ) # 保存下方便以后使用 #save(coadFilt,file = "./output/coadFilt.rdata") # 准备差异基因 load(file = "./output/coadDEGs.Rdata")- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
查看数据:
coadFilt[1:4,1:4] ## TCGA-NH-A8F7-06A-31R-A41B-07 TCGA-3L-AA1B-01A-11R-A37K-07 ## ENSG00000000003 15299 7257 ## ENSG00000000005 26 23 ## ENSG00000000419 5139 2058 ## ENSG00000000457 614 734 ## TCGA-4N-A93T-01A-11R-A37K-07 TCGA-4T-AA8H-01A-11R-A41B-07 ## ENSG00000000003 7125 2918 ## ENSG00000000005 67 89 ## ENSG00000000419 2626 844 ## ENSG00000000457 731 323- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
查看数据:
head(coadDEGs) ## logFC logCPM LR PValue FDR ## ENSG00000000419 1.018334 5.864185 58.75510 1.785702e-14 7.381441e-14 ## ENSG00000000460 1.423716 3.457955 153.99195 2.325351e-35 3.797553e-34 ## ENSG00000000971 -1.052867 4.847858 37.14551 1.096349e-09 3.067592e-09 ## ENSG00000001460 -1.006716 3.531623 170.11301 6.990106e-39 1.377268e-37 ## ENSG00000001497 1.151358 6.086819 114.28071 1.131077e-26 1.106818e-25 ## ENSG00000001617 1.167619 5.505217 51.08214 8.858051e-13 3.195640e-12 ## gene_name gene_type ## ENSG00000000419 DPM1 protein_coding ## ENSG00000000460 C1orf112 protein_coding ## ENSG00000000971 CFH protein_coding ## ENSG00000001460 STPG1 protein_coding ## ENSG00000001497 LAS1L protein_coding ## ENSG00000001617 SEMA3F protein_coding- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
我们用logFC最大的前500个基因演示:
top500 <- coadDEGs[order(abs(coadDEGs$logFC),decreasing =T),][1:500,]- 1
准备热图需要的表达矩阵:
heat.df <- coadFilt[rownames(coadFilt) %in% rownames(top500),] dim(heat.df)- 1
- 2
准备热图需要的样本信息,必须有一列和表达矩阵的列名相同:
coldata <- colData(data) dim(coldata) ## [1] 521 107 coldata.df <- as.data.frame(subset(coldata, select=c("barcode","sample_type","vital_status","gender", "ajcc_pathologic_t","ajcc_pathologic_n", "ajcc_pathologic_m"))) head(coldata.df) ## barcode sample_type ## TCGA-A6-5664-01A-21R-1839-07 TCGA-A6-5664-01A-21R-1839-07 Primary Tumor ## TCGA-D5-6530-01A-11R-1723-07 TCGA-D5-6530-01A-11R-1723-07 Primary Tumor ## TCGA-AA-3556-01A-01R-0821-07 TCGA-AA-3556-01A-01R-0821-07 Primary Tumor ## TCGA-AA-3660-11A-01R-1723-07 TCGA-AA-3660-11A-01R-1723-07 Solid Tissue Normal ## TCGA-AA-3818-01A-01R-0905-07 TCGA-AA-3818-01A-01R-0905-07 Primary Tumor ## TCGA-AA-3660-01A-01R-1723-07 TCGA-AA-3660-01A-01R-1723-07 Primary Tumor ## vital_status gender ajcc_pathologic_t ## TCGA-A6-5664-01A-21R-1839-07 Alive male T4a ## TCGA-D5-6530-01A-11R-1723-07 Alive male T2 ## TCGA-AA-3556-01A-01R-0821-07 Alive male T2 ## TCGA-AA-3660-11A-01R-1723-07 Alive female T3 ## TCGA-AA-3818-01A-01R-0905-07 Dead female T3 ## TCGA-AA-3660-01A-01R-1723-07 Alive female T3 ## ajcc_pathologic_n ajcc_pathologic_m ## TCGA-A6-5664-01A-21R-1839-07 N2a MX ## TCGA-D5-6530-01A-11R-1723-07 N0 M0 ## TCGA-AA-3556-01A-01R-0821-07 N0 M0 ## TCGA-AA-3660-11A-01R-1723-07 N0 M0 ## TCGA-AA-3818-01A-01R-0905-07 N0 M0 ## TCGA-AA-3660-01A-01R-1723-07 N0 M0- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
然后使用
TCGAvisualize_Heatmap函数画热图,其实也是complexheatmap的包装:TCGAvisualize_Heatmap(data = heat.df, col.metadata = coldata.df, cluster_rows = T, cluster_columns = T, scale = "row", extremes = seq(-2,2,1), color.levels = colorRampPalette(c("green", "black", "red"))(n = 5) )- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
会在当前目录生成一张热图:

火山图
使用所有基因的差异信息。
load(file = "./output/coadDEGsAll.Rdata") TCGAVisualize_volcano(x=coadDEGAll$logFC, y=coadDEGAll$FDR, # 纵坐标会自动变成-log10 x.cut = c(-1,1), y.cut = 2 )- 1
- 2
- 3
- 4
- 5
- 6
- 7
会在当前目录下保存火山图,这个图纵坐标太大了!

PCA图
rm(list = ls()) load(file = "./output/coadFilt.rdata") load(file = "./output/coadDEGsAll.Rdata")- 1
- 2
- 3
定义下样本类型:
# normal group1 <- TCGAquery_SampleTypes(colnames(coadFilt), typesample = c("NT")) # tumor group2 <- setdiff(colnames(coadFilt), group1)- 1
- 2
- 3
- 4
- 5
需要获得一个差异基因table:
# DEGs table with expression values in normal and tumor samples coadDEGsFiltLevel <- TCGAanalyze_LevelTab( FC_FDR_table_mRNA = coadDEGAll, typeCond1 = "Normal", typeCond2 = "Tumor", TableCond1 = coadFilt[,group1], TableCond2 = coadFilt[,group2] ) head(coadDEGsFiltLevel) ## mRNA logFC FDR Delta Normal ## ENSG00000161016 ENSG00000161016 1.0634492 4.608668e-12 74483.67 70039.71 ## ENSG00000167658 ENSG00000167658 0.4903969 2.389951e-05 65654.67 133880.68 ## ENSG00000137154 ENSG00000137154 0.6959504 2.038518e-08 62058.29 89170.56 ## ENSG00000089157 ENSG00000089157 0.7335765 1.574363e-09 55289.82 75370.22 ## ENSG00000108821 ENSG00000108821 2.7959748 6.188722e-22 55126.53 19716.39 ## ENSG00000111640 ENSG00000111640 0.9146113 1.156871e-12 54971.11 60103.24 ## Tumor start end ## ENSG00000161016 99007.33 144789765 144792587 ## ENSG00000167658 126985.97 3976056 3985463 ## ENSG00000137154 103264.10 19375715 19380236 ## ENSG00000089157 86214.63 120196699 120201235 ## ENSG00000108821 105676.55 50184101 50201632 ## ENSG00000111640 74969.70 6534512 6538374- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
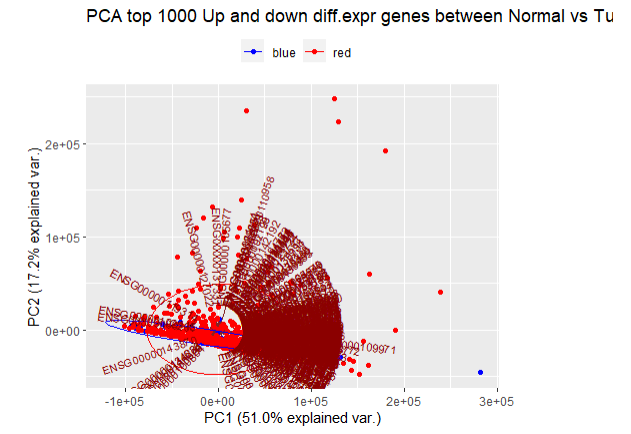
PCA分析并画图:
pca <- TCGAvisualize_PCA( dataFilt = coadFilt, dataDEGsFiltLevel = coadDEGsFiltLevel, ntopgenes = 1000, group1 = group1, group2 = group2 )- 1
- 2
- 3
- 4
- 5
- 6
- 7
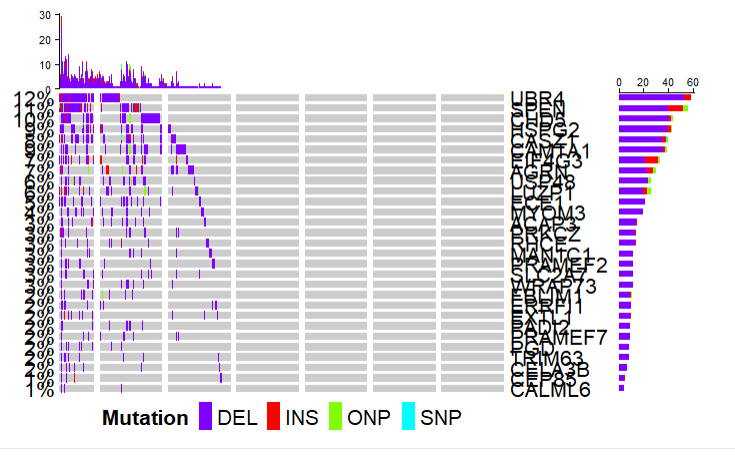
突变全景图
完全就是封装了
maftools包,并且帮助文档里还没更新,还写着TCGAquery_maf,但是这个函数在最新版本的TCGAbiolinks里面已经没有了。。rm(list = ls()) # 加载突变数据 load(file = "./TCGA-SNP/TCGA-COAD_SNP.Rdata") coad.maf <- data- 1
- 2
- 3
- 4
直接画就行,和
maftools一模一样,这里就不多介绍了,大家去用maftools吧。TCGAvisualize_oncoprint(mut = coad.maf, genes = coad.maf$Hugo_Symbol[1:30] )- 1
- 2
- 3
甲基化组间表达量/旭日图/条形图
可以参考之前的推文:
不得不说这些可视化函数优点鸡肋,不借助其他包是完全可以画出来图的,但是里面又都是封装的其他R包,而且还不如原装的R包好用!
-
相关阅读:
vue3+vite+ts中的@的配置
Redis 集群模式
Pytorch学习task02_待补
ssl教程易语言代码
vivo统一接入网关VUA转发性能优化实践
CSS 创建
基于JavaWeb的电影网站的设计与实现
T1077 统计满足条件的4位数(信息学一本通C++)
linux C++实现线程绑定CPU
家庭安全计划 挑战赛| 溺水预防
- 原文地址:https://blog.csdn.net/Ayue0616/article/details/127824325
